
Why reference pricing is the enemy of pharmaceutical innovation
Article published on Linkedin - May 13, 2025.
Jeff Coteur

January 2025: the perfect storm in healthcare policies?
Article published on Linkedin - January 21st, 2025.
Jeff Coteur

Inflation Reduction Act in the US: what do the CMS negotiated prices mean for clinical-stage biotechs?
Article published on Linkedin - August 25, 2024.
Jeff Coteur

Our policy on the use of AI in business conduct
Article published on Linkedin - May 26, 2024.
Jeff Coteur

Is the Parliament's position on the EU Pharma Package a good compromise?
Article published on Linkedin - Mar 21, 2024.
Jeff Coteur
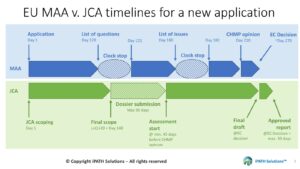
The draft Implementation act for the EU joint clinical assessment is out … so what?
Article published on Linkedin - Mar 9, 2024.
Jeff Coteur

US Senate Committee misses the mark on addressing the root cause of high list prices for drugs
White paper published on Linkedin - Feb 9, 2024.
Jeff Coteur
What the BeNeLuxA saga about Libmeldy® teaches us about value assessment & recognition for ATMPs
a white paper from iPATH Solutions™. Brussels, February 1st, 2024.
Last week, the Beneluxa initiative finally reached an agreement with the manufacturer for the reimbursement of the gene therapy Libmeldy in the three participating countries, Belgium, Netherlands and Ireland (https://beneluxa.org/news1). Beneluxa is often regarded as a flagship example of joint clinical assessment and joint pricing negotiation in Europe.
In the wake of the EU Joint Clinical Assessment (JCA) implementation for ATMPs planned in less than 12 months from now, the huge delays seen with the Beneluxa process for Libmeldy are certainly concerning. It took 3 years between EMA approval and reimbursement in the Beneluxa participating countries, this should be put into perspective with a 2.7 years median survival rate for children with late infantile metachromatic leukodystrophy -MLD- (https://pubmed.ncbi.nlm.nih.gov/20038527/) … how did we get to this situation?
First of all, the value assessment started late, took more than 1 year, and concluded of a clinical benefit “marginally greater [than BSC]” (https://beneluxa.org/archive#toc-11-october-2022-ireland-belgium-and-the-netherlands-complete-joint-hta-report-on-gene-therapy-libmeldy) while BSC in this case means palliative treatment. This contrasts with other countries in EU having conducted their own clinical assessment and coming to radically different conclusions: Germany’s GBA seeing a “considerable benefit” and France’s HAS a “moderate added value” (ASMR 3) for Libmeldy. This points to the need for value assessment frameworks that are adapted to the challenges of generating evidence for ATMPs which are often studied in single-arm, uncontrolled studies and relying on external/historic control arms.
Secondly, pricing negotiations took also more than 1 year. While not privileged to the negotiation discussions, having a poor recognition of the intrinsic value of a medicine is most probably one of the main root causes for this delay. For instance, ICER in the US determined that the health-benefit price benchmark (iow. the price that is justified by expected health benefits) for Libmeldy would be $2.3M - $3.9M (https://icer.org/), while the list price in several EU countries falls within or below that range. Another obvious challenge is the affordability of such high single upfront costs while the therapy provides health benefits spread over a patient’s lifetime. Several payer institutions are leading the way in developing payment frameworks (eg. Payments based on actual outcomes at patient- or population-level) that are more adapted to the challenges of ATMPs. For instance, CMS recently developed an access model for the implementation of outcomes-based agreements by state Medicaid agencies (see ValueGenome linkedin post about this, https://www.linkedin.com/posts/valuegenomellc_cell-and-gene-therapy-cgt-access-model-activity-7158489321669353472-Flzs?utm_source=share&utm_medium=member_desktop ).
With the number of approvals for ATMPs expected to grow significantly in the future, our healthcare ecosystem needs to adapt to the specific challenges of these therapies in terms of value assessment and recognition. This will contribute to plugging the access gap for these life-saving and life-changing therapies.
Jeff Coteur – iPATH Solutions™

Will Rare Diseases Become Orphan Again? Orphan Drugs Development Faces Uphill Battle with New Regulatory and Access Policy Changes
White paper published on Linkedin - Nov 21, 2023.
Geoffroy Coteur & Anja Pownell

Unmet need for supplemental influenza prevention beyond vaccination: IDWeek poster to be presented
Post published on Linkedin - Oct 11, 2023.
Geoffroy "Jeff" Coteur
